(adapted from section 2, "Respiratory, postural and spatio-kinetic motor stabilization, internal models, top-down timed motor coordination and expanded cerebello-cerebral circuitry: a review".)
Paleoanthropologically knapping
dates back to 2.5 mya (Semaw, Renne et
al. 1997), though the example examined below that allows the
reconstruction of
the manufacture of such stone tools is young at ca. 2.34 mya (Delagnesa
and
Roche 2005). In knapping, an individual holds a core stone in the
nondominant
hand that they strike with a hammerstone held in their dominant one to
produce
flakes and a modified core (fig. 1.). Reconstruction of surviving
flakes and
cores shows (Delagnesa and Roche 2005) that this was done in terms of
an
overall knapping action plan (see fig. 2.). There has also been recent
studies
upon contemporary people as to the motor and the neurological processes
involved (Marzke, Toth et al. 1998; Stout, Toth et al. 2000; Marzke
2006; Roux
and David 2006; Stout, Toth et al. 2008).
Knapping is illustrated using
the reconstructed knapping stones found at
Lokalalei 2C (
Need for
impact stabilization
The success of knapping, first,
has been found to depend crucially upon
the hammerstone hitting an appropriately stabilized core.
Electromyography
research shows that there needs to be a millisecond timed scheduled
stiffness
adjustment to stabilize the hand holding the core that prepares the
hand for
the otherwise perturbing force of the hammerstone’s impact (Marzke,
Toth et al.
1998) (Fig 2 A and B). At the moment of impact, a coordinated loosening
of hand
grasp is also needed to protect the hand that “is brief enough that it
does not
allow displacement of the tool [hammerstone]” (Marzke 2006: p. 248). If
this
timed anticipatory stiffness is prepared/relaxed incorrectly, not only
could
the hand be injured but also the core will not be stabilized when hit,
and as a
result—a defective flake or none—will be produced.
Need for
overall manufacture plan
Second, knapping critically
depends upon the executive formulation of an
overall manufacture plan (Roux and David 2006) and so abstract
projectuality (Amati
and Shallice 2007) (see fig. 2). That is (i) having before
commencement, a
notion of the final product, (ii) an ability to treat each knap as
intermediate
stage to such a product, and (iii) capacity to modify the force,
position and
manner of each knap in terms of the changing debitage of the core to
achieve
the intended product (the knaps in fig. 2 A and C). Since cores can
need
secondary modification to enable knapping, and knaps can fracture the
core in
nonintended ways, it also requires, (iv) skill to identify errors and
adjust
new knaps to correct for them within the overall product making plan
(as in
fig. 2 B). Thus, the motor faculty of knapping requires cognitive
specializations
such as sustained attention, error detection, short- and long-term
memory,
action set shifting, and the hierarchization of ultimate and substage
motor
goals. For instance, the sequence illustrated in fig. 2 upon the
refitted group
core stone 2 found at Lokalalei 2C (Delagnesa and Roche 2005) could not
have
been done—as they were—2.34 myr by a brain that lacked the ability to
organize
its motor actions by such executive-type functions.
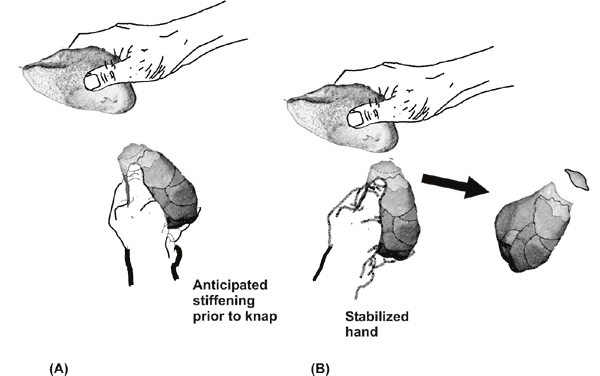
Fig
1. Knapping
is illustrated using the reconstructed knapping stones found at
Lokalalei 2C (
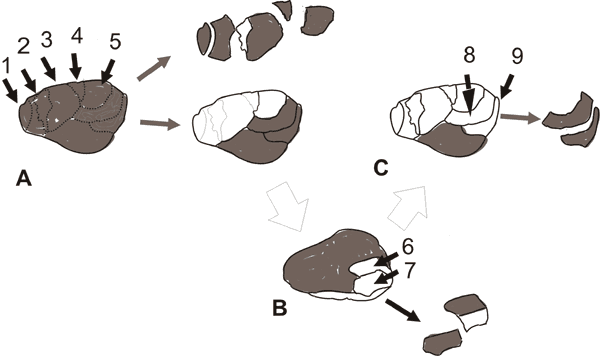
Fig 2. The refitting group core stone 2 was
knapped into nine flakes. This happened in three stages. (A) five
flakes, 1, 2,
3, 4 and 5 were knapped. (B) The knapper reshaped the core by removing
a
protrusion on the other side of the stone, ( 6 and 7 flakes) so
creating an
edge that was used (C) to make two more knaps, 8 and 9 (Delagnesa and
Roche
2005).
Human uniqueness
Stone tool
making in nonhuman primates
No animal apart from Homo
can anticipatorily adapt in a time
accurate manner the stiffness in the core holding hand in regard to the
hammerstone’s impact force, nor engage in the executive function-like
modification of motor control guided by the use of an action plan.
Attempts to
tutor the bonobos, to knap, for example, have been without success
(Schick,
Toth et al. 1999). This is not because they cannot use motor control to
modify
stones, nor because they lack the idea to make tools. Bonobos, such as
Kanzi and
Panbanisha are able to grasp the idea of stone modification—but they
are only
able to engage in this (even when shown how to properly knap) through
(i) an
initial uncontrolled “thrust percussive” method of throwing and braking
stones
on the ground, and (ii) later with more experience by a stabilization
using the
ground (Davidson and McGrew 2005, see fig. 3 on page 800 in which
Panbanisha
holds a stone against the ground with
her left hand). The former method of stone modification is also found
in wild
chimpanzees (Boesch and Boesch 1990; Mercader, Barton et al. 2007).
Both types
of “thrust percussive” tool making is characterized by not needing the
anticipatory coordination of the two hands, nor highly complex action
planning.
Byrne (2005, p. 166) has noted
“it may be very well that no living great
ape is capable of learning the
motor
skill involved in aiming a powerful and accurate blow at an object held
in the
other hand: it is the combination that may be beyond them, because
there is no
doubt that living apes have both great limb power and delicate
precision, in
separate contexts”. Here this inability is identified as the lack of a
capacity
to coordinate energetic force in one hand, while the other engages in
accurately timed adjusted stabilization against its quick forceful
impact.
Reasons for
human uniqueness
What has limited nonhuman
primates from possessing the above noted motor
dexterity needed to knap? One explanation might be human specific hand
anatomy:
(i) human fingers have optimal length to work with the human thumb
(Napier
1960), (ii) a stable and extended pulp region exists on the human thumb
for
aiding firm precision pinches and object holding (Susman 1994), and
(iii) human
wrist bones allow for a power grip (Marzke, Wullstein et al. 1992).
These
musculoskeletal adaptations while optimizing the human hand for
knapping,
however, are not responsible for creating the motor capacity to
anticipatorily
stiffen effectively the core holding hand. In addition, human hand
anatomy
contrary to “long standing” assumptions is not completely unique to the
human
species as: “each of the features forming the human morphological
pattern
appears variably in at least one or more nonhuman primate species”
(Shrewsbury,
Marzke et al. 2003: p. 41). Nor is refined finger control peculiar to
humans:
young chimpanzees, for
example, appear to have more individual control over their fingers than
humans (Landsmeer 1993: p. 330).
Novelty of human motor control
and knapping
The human specific motor
control that underlies knapping is critically
dependent upon its capacity to be organized within accurate time-based
internal
models.
˜
First, these internal
models allow the refined time prediction of the
kinematics and kinetics of the musculoskeletal system of the upper body
so that
motor control can in a non-stereotypical timed manner stabilize
“stiffness” in
one motor element (the core holding arm) in regard to another
independent motor
element (the striking hammerstone) (Marzke, Toth et al. 1998).
˜
Second, such internal
models allow the predictions needed to modify each
temporally successive knap/stabilization in terms of the requirements
of a complex
action plan.
˜
Third, a factor
not discussed in depth due to lack of space is aiding the
generation of the intersegmental dynamic coordination of forces in the
dominant
hammerstone holding arm (Sainburg and Kalakanis 2000; Sainburg 2002;
Wang and
Sainburg 2007) that is needed to accurately target the hammerstone and
its
strike impact on the core stone. Though the emphasize in this paper is
upon
stabilization, internal models also play a significant role in
enhancing the
voluntary capacities of musculoskeletal action.
In regard to motor ability,
hand anatomy is secondary to the primacy of
such human specific motor competence.
Nonhuman
specific motor stabilization and its
limits
Innovations to provide this motor
control are needed if humans are to knap since from a biological
perspective,
the submovement stabilization of the core holding hand cannot be timed
in a
task-determined manner by the already existing stabling processes found
in
nonhuman vertebrates such as preflexes and spinal adjustment reflexes.
Preflexes and spinal adjustment
reflexes embedded locally in the body’s
musculature underlie the motor stabilization of movements in nonhuman
animals. Thet
are unlike the anticipatory motor adjustment of proficiency since these
can
only be acquired through prolonged practice when mastering motor
expertise in a
particular task. Instead, preflexes and spinal adjustment reflexes are
evolutionarily precustomized to provide specific stabilizations for (i)
frequently
encountered, or (ii) survival critical perturbations, that are faced
during the
execution of (iii) an evolutionarily limited repertoire of highly
adapted
stereotypical motor movements. This makes such musculoskeletal embedded
stabilization mechanisms functional only for a few highly evolved motor
employments of the musculoskeletal system, not task-determined motor
skills
that require prolonged mastery.
Such lower-level
musculoskeletal stabilization mechanisms are not able to
offer the constantly modified time-based feedforward motor
stabilization needed
for knapping (and more generally, other practice-dependent forms of
expert
dexterity). First, when the two stones strike, it requires a task
specific (not
stereotypic) timed anticipatory coordination between the two hands that
takes
many years of practice and learning to master. Second, this
anticipatory and
timed adjustment must be situationally highly tailored as it is
successively
modified for each knap in terms of action goals (given the core’s
changing
shape and intended core debitage). Third, knapping illustrates only one
of the
many different kinds of hand stabilizations that humans acquired
through
prolonged practice as they make and use diverse artifacts. Other kinds
of motor
stabilization with different task-determined time accurate
coordination, for
example, were needed by early Homo for the expert
usage and making of
tools that due to perishable materials—wood, bone, skin—are not
preserved.
Knapping and
internal models
The anticipatory stabilization
needed for knapping reflects the existence
in humans of the evolution of an expanded neurobiological capacity for
(1)
making time-organized internal predictive models of the kinetics,
kinematics
and action organization, and (2) the integration of them needed to
engage in
complex and practiced tasks.
The increased ability in Homo to practice and
refine such models allows Homo
to engage in motor control that can for each occasion be exact and
proficient
in its anticipatory adjustments. As a result, motor control can
stabilize
effectively its actions to produce constant motor effects in spite of
changing
circumstances such as when a hammerstone hits a core stone of changing
weight
and shape. This top-down motor timed adaptation based upon internal
models that
characterizes proficient human motor control underlies not only for the
expert
and dexterous use of the hands (as in knapping) but also human
bipedality and human song/speech vocalization.
Although not discussed in a
paleoanthropological context, internal model
processes are well researched, especially in regard to their
implementation in
the circuits between the cerebellum and the cerebral cortex (parietal,
motor,
premotor and prefrontal areas) (Kawato and Gomi 1992; Wolpert and
Kawato 1998;
Doya 1999; Wolpert, Doya et al. 2003; Ito 2006; Ito 2008). Such
top-down
internal model based motor adaptation is paleoanthropologically
important
because these circuits in humans are uniquely expanded compared to
those in
nonhuman primates (MacLeod, Zilles et al. 2003), particularly in regard
to the
prefrontal cortex (Ramnani 2006; Ramnani, Behrens et al. 2006), and in
preliminary evidence that H. sapiens sapiens under
went specific
cerebellum enlargement at the cost of overall cerebral hemisphere size
(Weaver
2005). They are, moreover, known from studies upon cerebellar agenesis
to be
involved in dexterity (Nowak, Timmann et al. 2007), and from
neuroimaging to be
activated during knapping (Stout, Toth et al. 2000; Stout, Toth et al.
2008).
Further, humans have a
prolonged nonadult stage in development,
“adolescence” in which the relationship between gray matter and its
connective
white matter in the brain changes (Lenroot and Giedd 2006). These
changes can
be specified linked to hand motor skill mastery. Learning to play the
violin to
an expert level thickens the connections between the two cerebral
hemispheres (Schlaug,
Jancke et al. 1995), and acts to increase nearly threefold the area
devoted to
the left hand (Elbert, Pantev et al. 1995). In keyboard players, there
is an
enlargement of the motor cortex that is proportional to the number of
years
that they have been practicing their instrument (Amunts, Schlaug et al.
1997).
Musicians also have slightly expanded cerebellums—a difference that
increases
with the number of years that they have been practicing (Hutchinson,
Lee et al.
2003). Consistent with these motor skill mastery changes in the brain
linking
to enhanced internal models, there is increased accuracy during
adolescent in
the internal models of hand movements, as well as improved fine-motor
skills (Choudhury,
Charman et al. 2007).
Knapping,
human uniqueness, and motor control
summary
Knapping illustrates four
things.
First, that humans can
proficiently make actions that other animals
cannot.
Second, that though
anatomy helps (the human hand is optimized for a
power grip), this is not the explanation: what is critical is the timed
adjustment and coordination of different movement elements made
possible by
time-exact internal models, a form of motor stabilization that cannot
be done
by the locally embedded preflexes and spinal adjustment reflexes that
underlie
the motor control of nonhuman animals. These models, moreover, provide
the
basis for the temporal and sequential goal hierarchization that allows
for
different body movements to be organized as part of a task action plan.
Third, that the
opportunity to base motor control upon such internal
models depends upon something unique to Homo—expanded
cerebello-cerebral
cortex circuits including those with prefrontal areas, and also the
existence
of a prolonged nonadult maturation stage needed to refine and integrate
them
together as expert motor skills (Skoyles 2008).
Fourth, knapping, it can
be added, is of great adaptive advantage (it
provides sharp cutting implements essential for the extraction of
high-energy
foods such as bone marrow) (Dominguez-Rodrigo, Pickering et al. 2005).
This
makes the origin of the internal model based motor stabilization
central to any
understanding of human origins. This is particularly so—if, as proposed
below—that such internal models also underlie the biological
distinctive
character and utility of human bipedality and human vocalization
Knapping, thus, shows that
there is an evolutionary link between changes
in motor stabilization mechanisms (the shift from preflexes and spinal
adjustment reflexes to accurately task timed anticipatory adjustments),
and the
evolution of what is peculiar to humans. Further, that this shift,
identifies
processes that are scientifically already well experimentally
investigated.
References
Amati, D. and T. Shallice (2007). "On the
emergence of modern
humans." Cognition 103: 358-385.
Amunts, K., G. Schlaug, et al. (1997). "Motor
cortex and hand
motor skills: Structural compliance in the human brain." Human
Brain
Mapping 5: 206 - 215.
Boesch, C. and H. Boesch (1990). "Tool use and
tool making in
wild chimpanzees." Folia Primatol (
Byrne, R. W. (2005). The maker not the tool:
The cognitive
significance of great ape manual skills. Stone Knapping: the
necessary
conditions for a uniquely hominid behaviour. V. Roux and B.
Bril.
Cambridge, McDonald Institute: 159-169.
Choudhury, S., T. Charman, et al. (2007).
"Development of
action representation during adolescence." Neuropsychogia
45:
255-262.
Davidson,
Delagnesa, A. and H. Roche (2005). "Late
Pliocene hominid
knapping skills: The case of Lokalalei 2C,
Dominguez-Rodrigo, M., T. R. Pickering, et al.
(2005).
"Cutmarked bones from Pliocene archaeological sites at Gona, Afar,
Doya, K. (1999). "What are the computations of
the cerebellum,
the basal ganglia and the cerebral cortex?" Neural Netw
12(7-8):
961-974.
Elbert, T., C. Pantev, et al. (1995).
"Increased cortical
representation of the fingers of the left hand in string players." Science
270: 305-7.
Hutchinson, S., L. H. Lee, et al. (2003).
"Cerebellar volume of
musicians." Cerebral Cortex 13(9): 943-9.
Ito, M. (2006). "Cerebellar circuitry as a
neuronal
machine." Prog Neurobiol 78(3-5): 272-303.
Ito, M. (2008). "Control of mental activities
by internal
models in the cerebellum." Nat Rev Neurosci 9(4):
304-13.
Kawato, M. and H. Gomi (1992). "A computational
model of four
regions of the cerebellum based on feedback-error learning." Biol
Cybern 68(2): 95-103.
Landsmeer, J. M. (1993). Evolution and the
hand. Hands of
primates. H. Preuschoft and D. J. Chivers. Wien,
Springer-Verlag: 323-333.
Lenroot, R. K. and J. N. Giedd (2006). "Brain
development in
children and adolescents: insights from anatomical magnetic resonance
imaging." Neurosci Biobehav Rev 30(6): 718-29.
MacLeod, C. E., K. Zilles, et al. (2003).
"Expansion of the
neocerebellum in Hominoidea." J Hum Evol 44(4):
401-29.
Marzke, M. W. (2006). Who made stone tools? Stone
knapping: The
necessary conditions for a uniquely hominin behaviour. V.
Roux and B.
Brill. Cambridge, McDonald Institute: 243-255.
Marzke, M. W.,
Marzke, M. W., K. L. Wullstein, et al. (1992).
"Evolution of
the power ("squeeze") grip and its morphological correlates in
hominids." Am J Phys Anthropol 89(3): 283-98.
Mercader, J., H. Barton, et al. (2007).
"4,300-year-old
chimpanzee sites and the origins of percussive stone technology." Proc
Natl Acad Sci U S A 104(9): 3043-8.
Napier, J. R. (1960). "Studies of the hands of
living
primates." Proceedings of the Zoological Society of
Nowak, D. A., D. Timmann, et al. (2007).
"Dexterity in
cerebellar agenesis." Neuropsychologia 45: 696-703.
Ramnani, N. (2006). "The primate
cortico-cerebellar system:
anatomy and function." Nat Rev Neurosci 7(7):
511-22.
Ramnani, N., T. E. Behrens, et al. (2006). "The
evolution of
prefrontal inputs to the cortico-pontine system: diffusion imaging
evidence
from Macaque monkeys and humans." Cereb Cortex
16(6): 811-8.
Roux, V. and E. David (2006). Planning
abilities as a dynamic perceptual-motor
skill: An actualist study of different levels of expertise involved in
stone
knapping. Stone knapping: The necessary conditions for a
uniquely hominin
behaviour. V. Roux and B. Brill. Cambridge, McDonald
Institute: 91-108.
Sainburg, R. L. (2002). "Evidence for a
dynamic-dominance
hypothesis of handedness." Exp Brain Res 142(2):
241-58.
Sainburg, R. L. and D. Kalakanis (2000). "
Differences in
control of limb dynamics during dominant and nondominant arm reaching."
Journal
of Neurophysiology 83: 2661-2675.
Schick, K. D.,
Schlaug, G., L. Jancke, et al. (1995).
"Increased corpus
callosum size in musicians." Neuropsychologia
33(8): 1047-55.
Semaw, S., P. Renne, et al. (1997).
"2.5-million-year-old stone
tools from
Skoyles, J. R. (2008). "Human metabolic
adaptations and
prolonged expensive neurodevelopment: A review." Nature
Preceedings http://hdl.handle.net/10101/npre.2008.1856.1.
Stout, D.,
Stout, D.,
Susman, R. L. (1994). "Fossil evidence for
early hominid tool
use." Science 265: 1570-1573.
Wang, J. and R. L. Sainburg (2007). "The
dominant and
nondominant arms are specialized for stabilizing different features of
task
performance." Exp Brain Res 178(4): 565-70.
Weaver, A. H. (2005). "Reciprocal evolution of
the cerebellum
and neocortex in fossil humans." Proc Natl Acad Sci U S A
102(10):
3576-80.
Wolpert, D. M., K. Doya, et al. (2003). "A
unifying
computational framework for motor control and social interaction." Philosophical
Transactions of the Royal Society of
Wolpert, D. M. and M. Kawato (1998). "Multiple paired forward and inverse models for motor control." Neural Netw 11(7-8): 1317-1329.